Michigan pauses use of Johnson & Johnson COVID-19 vaccines 'out of an abundance of caution'

Audio By Carbonatix
[
{
"name": "GPT - Leaderboard - Inline - Content",
"component": "35519556",
"insertPoint": "5th",
"startingPoint": "3",
"requiredCountToDisplay": "3",
"maxInsertions": 100,
"adList": [
{
"adPreset": "LeaderboardInline"
}
]
}
]
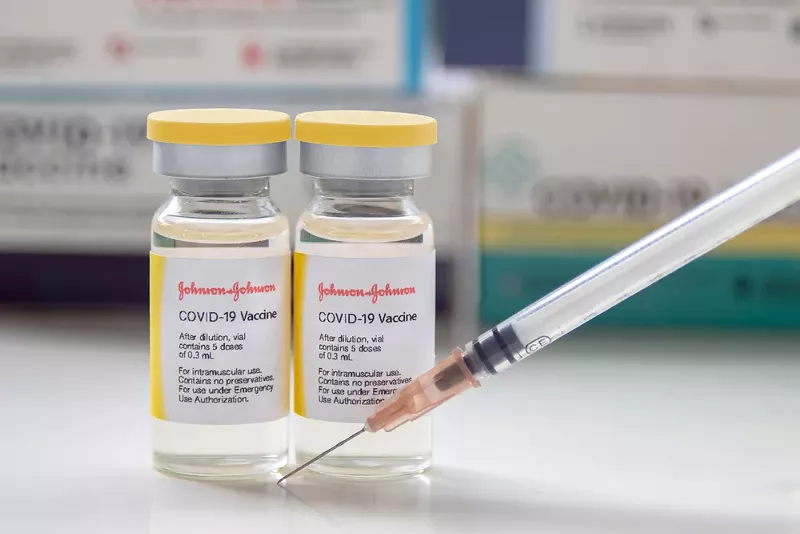
Following the recommendations from the Food and Drug Administration and Centers for Disease Control and Prevention, the Michigan Department of Health and Human Services is calling for a temporary pause of the use of the Johnson & Johnson's single-dose COVID-19 vaccines after a small number of people experienced blood clots.
Dr. Joneigh Khaldun, the state's chief medical executive and chief deputy for health, said the pause was out of "an abundance of caution" while the issue is investigated. The issue appears to be very rare: Out of more than 6.8 million doses administered in the U.S., only six reports of blood clots have surfaced.
"As we learn more about this from our federal partners, we will update vaccine providers and Michiganders across the state," Khaldun said in a statement. "We encourage everyone to continue making appointments to be vaccinated with the safe and effective Pfizer and Moderna COVID-19 vaccines at this time. These vaccines are the way we are going to end this pandemic as quickly as possible and move toward a sense of normalcy."
Johnson & Johnson has responded to concerns, as reported by ABC News.
"We are aware that thromboembolic events including those with thrombocytopenia have been reported with COVID-19 vaccines," the company said. "At present, no clear causal relationship has been established between these rare events and the Janssen COVID-19 vaccine. We continue to work closely with experts and regulators to assess the data and support the open communication of this information to healthcare professionals and the public."
All six cases occurred among women between the ages of 18 and 48, and symptoms occurred six to 13 days after vaccination. While an anticoagulant drug called heparin is typically used to treat blood clots, officials worry heparin may be dangerous in this situation, and are seeking alternative treatments.
Michigan has administered nearly 200,000 doses of the one-shot Johnson & Johnson vaccine, according to state data.
Anyone who received a Johnson & Johnson vaccine and experienced a severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider.
The CDC is holding a meeting on Wednesday about the issue.
The vaccines have not been FDA-approved, but the FDA issued emergency-use authorization due to many clinical trials that have found them to be effective in preventing COVID-19. While the news may deter people who were already skeptical of the vaccines, officials say it is actually proof that the vaccines are being thoroughly vetted.
Plus, to put it in perspective, birth control pills can also cause blood clots in women at a much higher rate (1 in 3,000), than officials are reporting for the Johnson & Johnson vaccine (1 in 1,000,000).
"As someone who got the J&J vaccine 8 days ago, and who took oral contraceptives for 20 years, I’ll take these odds," Dr. Angela Rasmussen, a virologist from Washington, wrote on Twitter.
The news comes as Detroit launched a "Neighborhood Vaccination Week" on Monday that was going to bring the Johnson & Johnson shots to local clinics to vaccinate people quickly in their own communities. In the meantime, the city says it will now offer the two-dose Moderna and Pfizer vaccines instead, which are not impacted by the pause, according to The Detroit News.
The Johnson & Johnson vaccines were also planned to be used during the final two weeks of the massive FEMA-operated vaccination site at Detroit's Ford Field in May.
Stay on top of Detroit news and views. Sign up for our weekly issue newsletter delivered each Wednesday.






